Description
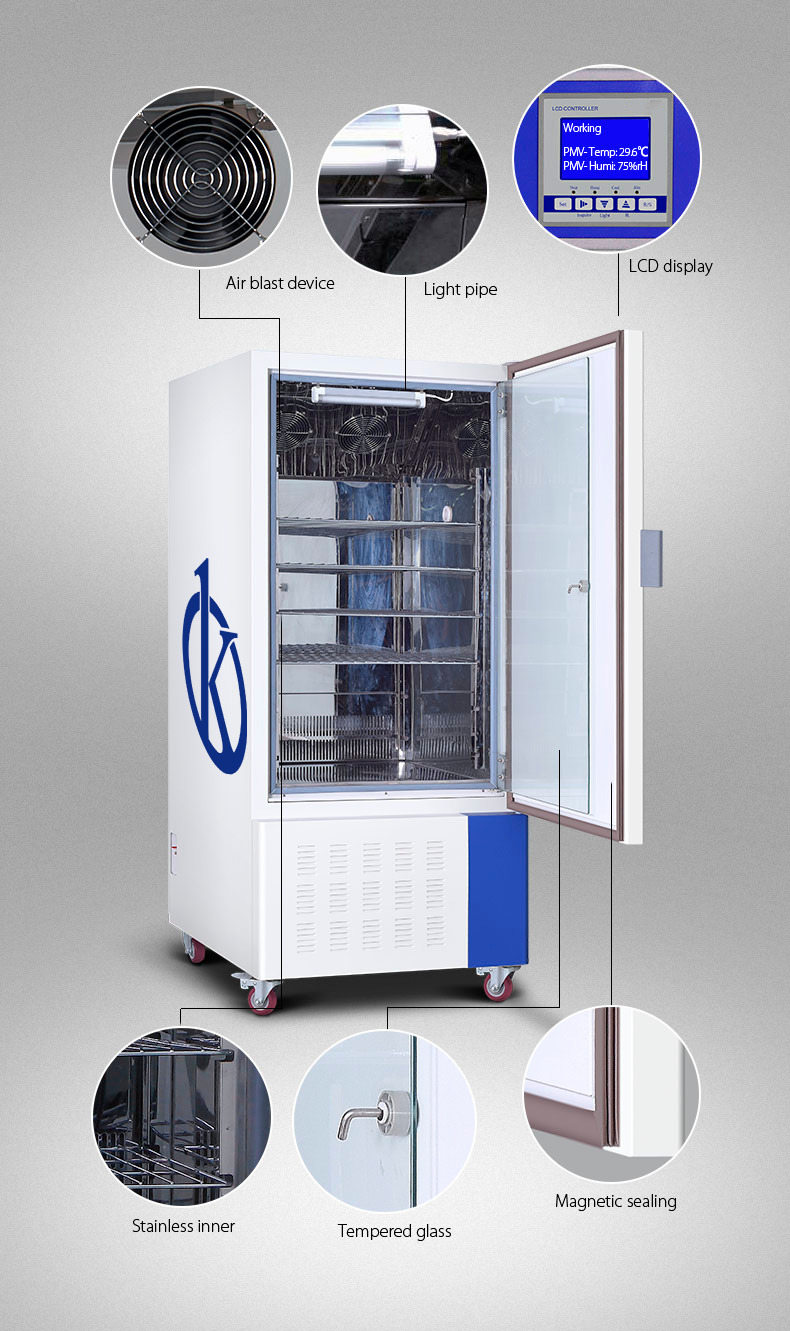
The Drug Stability Test Chamber YR05350 // YR05352 is an essential tool for the pharmaceutical, biotechnology, and food industries, offering precise control over environmental conditions such as temperature and humidity. It is equipped with a world-class compressor, auto-defrost function, and an advanced programmable PID control system to ensure accurate and reliable operation. The chamber’s ergonomic design, excellent imported sensors, and innovative humidification system make it a standout choice for laboratories and research facilities needing consistent and stable testing environments.
Market Price
The Drug Stability Test Chamber is a high-precision instrument that typically ranges between $9,500 and $9,800 in the market. This price range reflects the specialized features and advanced technology it offers, making it a worthwhile investment for organizations requiring reliable environmental simulation for drug stability testing.
Frequently Asked Questions
- What industries benefit from using this test chamber? The chamber is ideal for the pharmaceutical, biotechnology, and food industries, particularly where precise environmental control is crucial.
- What is the power supply requirement for the chamber? It requires an AC220V/50Hz power supply.
- Can the chamber be connected to multiple devices? Yes, it supports connectivity with up to 16 units for comprehensive monitoring and control.
Advantages and Disadvantages
Advantages: The chamber offers precise temperature and humidity control, energy efficiency, and a robust security system, ensuring reliable performance over prolonged periods. Its ergonomic design facilitates ease of use and maintenance.
Disadvantages: The initial investment cost may be high for smaller laboratories, and the setup may require specialized installation expertise.
Use of the Product in the Field
This chamber is commonly used in pharmaceutical laboratories for testing drug stability under various environmental conditions. Its precise control over temperature and humidity allows researchers to simulate real-life storage conditions, ensuring that products meet regulatory standards before hitting the market.
Recommendations
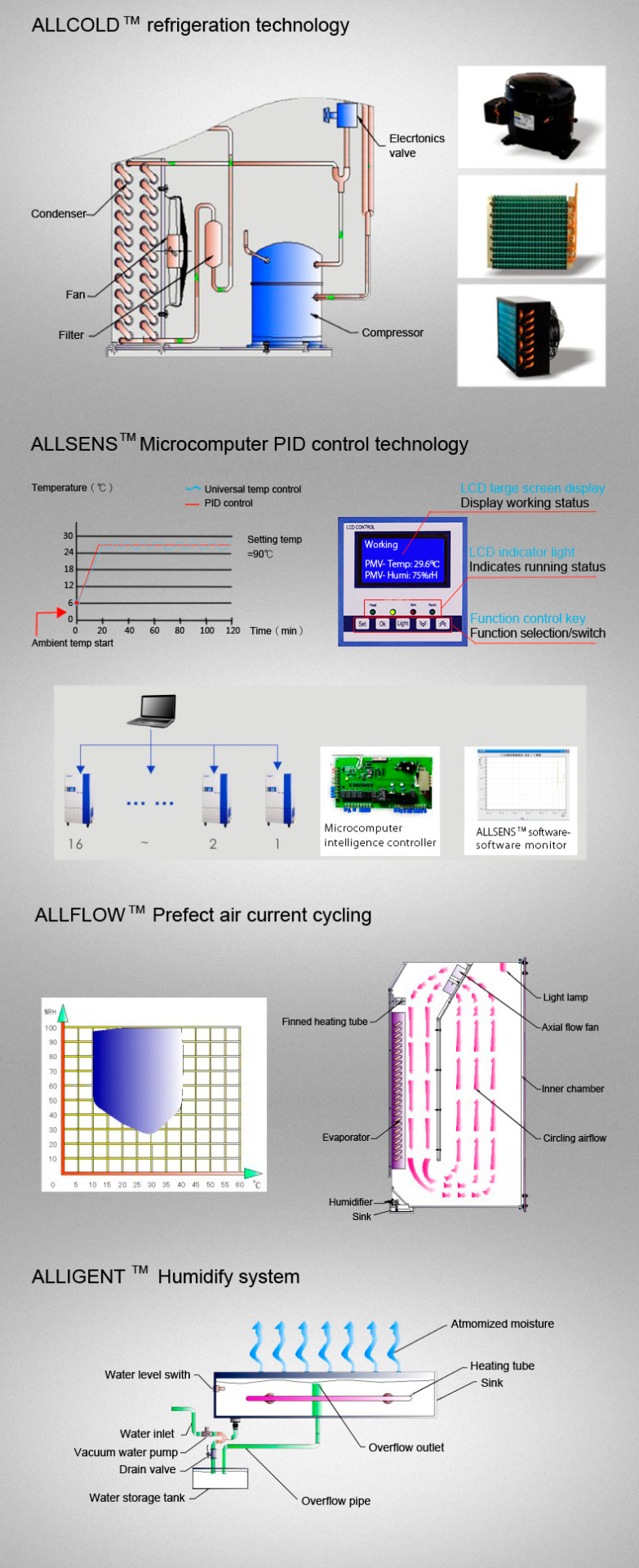
For optimal performance, regularly calibrate the chamber’s sensors and utilize the ALLSENSTM software for remote monitoring. Ensure the unit is placed in a stable environment where the ambient temperature ranges between 10-30°C and humidity is below 70% to maintain its longevity.
Features
- Balanced refrigeration technology with auto-defrost.
- 30-stage microprocessor PID controller.
- Forced convection for uniform temperature.
- Adaptive PID controller to prevent temperature fluctuations.
- Optional ALLSENSTM software for remote control.
Technical Specifications
|
Product Model |
ALLIGENT-KG Comprehensive drug stability test chamber |
||
|
YR05350 |
YR05351 |
YR05352 |
|
|
Convection Mode |
Forced Convection |
||
|
Control System |
Thirty stages Microprocessor PID controller |
||
|
Temp. Range |
0~60 |
||
|
Temp. Accuracy |
0.1℃ |
||
|
Temp. Fluctuation (10-40℃) |
±0.5℃ |
||
|
Temp. Uniformity(10-40℃) |
±1 |
±1 | ±1.5 |
|
Humidity Range |
Humidity Range:50~90%RH,Humidity Fluctuation:±3%RH |
||
|
Working environment |
Ambient temperature:10~30℃, Humidity <70% |
||
|
Insulation materials |
Imported environmental protection type material |
||
|
External Dimensions (H×W×D) |
1410×650×680 |
1730×650×740 |
1700×745×930 |
|
Internal Dimensions (H×W×D) |
760×510×390 |
1100×510×450 |
1050×600×640 |
| Interior Volume(L) |
150 |
250 |
400 |
|
Interior steel materials |
Interior steel materials |
||
|
Power Consumption (W) |
1080 |
1100 |
1350 |
| Power supply |
AC220V/50Hz |
||
| Net Weight(KG) |
107 |
135 |
158 |
| Shipping Weight(KG) |
132 |
162 |
186 |
| Shipping Dimensions (H×W×D) |
1610×750×830 |
1930×750×890 |
1900×840×1080 |